We all know that regular health checks are vital. Luckily, today, medical devices are not only in doctors’ offices but also in our pockets. How did it happen?
New technologies have created a new health workflow that gives patients and medical personnel much more opportunities. Software-as-a-medical device (SaMD) allows you to replace some diagnostic equipment with familiar devices like smartphones and smartwatches.
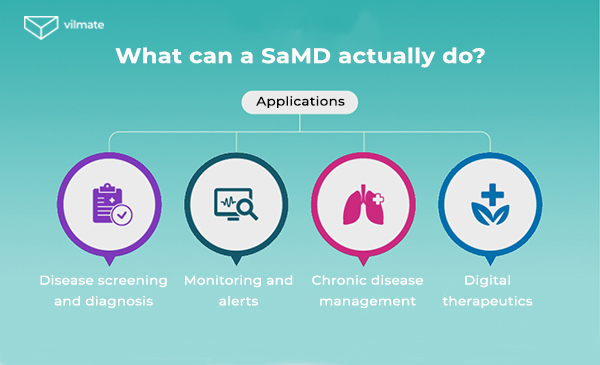
So, in this article, we’ll discuss medical device software development, its main stages, and purposes. We will try to understand why it is important to find the right audience and also look at examples of successful SaMD.
Who needs SaMD?
SaMD is designed for devices that everyone has. This is the difference between SaMD and software for medical devices, which is associated with medical devices only. For example, to get the work of MRI machines. Software for medical devices is not an independent product and is relevant only in the professional field.
So, research on the target audience is fundamental. It will help you grab the attention of a larger audience and be more useful for them. It’s worth noting that each feature takes time and money to develop, so there should be no useless things left in the project.
Each target group needs different features of the medical device software. Thus, choosing the right target group is vital for your medical product. Also, each option should be developed with the involvement of dedicated specialists since we are talking about health.
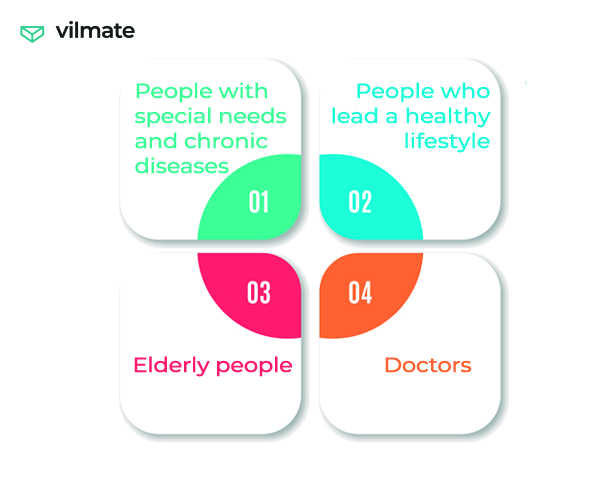
Everyone cares about their health, someone more, someone less. And the large target audiences of SaMD are:
How can Software-as-a-medical device help?
One Apple a day keeps a doctor away. The proverb took on a new meaning when the Apple Watch appeared. And nowadays, smartwatches are one of the most popular wearables categories. According to Statista, this handy technology is estimated to reach more than a quarter of a billion devices shipped by 2025.
Smartwatch is the most convenient and compact device for medical product development. They are equipped with many sensors that can measure important health indicators. So the developers have released many useful applications for different audiences.
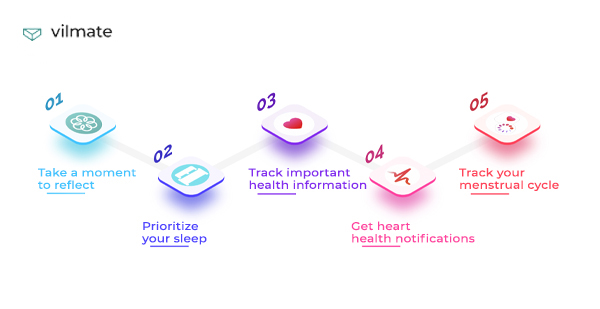
People who lead a healthy lifestyle appreciate sports training, the ability to count calories, and an ECG sensor.
After the COVID-19 pandemic, many things changed. And things like measuring the level of oxygen in the blood and a program that helps wash hands have become so much more relevant.
If you care about your emotional health, try sleep monitoring or the Mindfulness app. It will help you do breathing exercises and meditate. Breathe, please!
How can Software-as-a-medical device save lives?
In a smartwatch device, you can find patient safety features like Heart Rate, Mindfulness, Sleep, Workout, Blood Oxygen, Noise, and others.
The device can remind you to move if you sit for too long. The application can suggest a breathing exercise if the pulse has risen because of stress. It’ll also warn about high noise levels that may damage your hearing. That’s why smartwatches hold great promise for Software-as-a-medical device development.
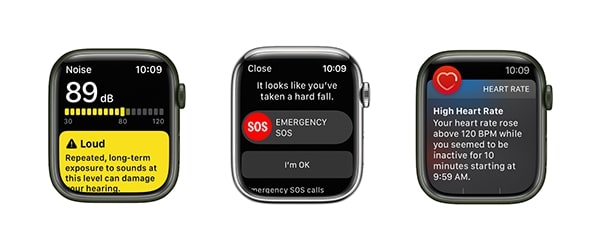
SaMD can save lives not only in theory but also in practice. For example, the fall detection sensor on the Apple Watch can call an ambulance if the user has lost consciousness. It’s especially true for the elderly and their families.
SaMD concerns mainly diagnostic medical devices. It can warn about problems with the heart and breathing in time and notice sleep apnea. Diagnostic data must be immediately transferred to the doctor.
As soon as we’ve realized what Software-as-a-medical device can bring and for whom, now we’re ready to discuss the main stages of the SaMD product development process.
Main stages of SaMD development
The medical device software development process consists of project creation, actual development, quality assurance, integration, and maintenance.
You can find the main stages in the image below.
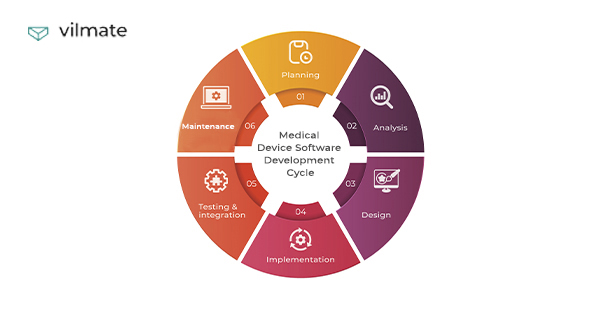
-
Product discovery & software development planning
It’s not easy to make a new start, but a qualified software development company can be of great help there. Planning requires careful analysis and professional advice. Your medical device software partner can analyze the market demand and assist in defining and prioritizing the needed functionality for your product.
The reasoning is quite simple. Are you willing to pay for fancy features that won’t add much business value? So product discovery will help you significantly reduce the risk of building an expensive product no one needs.
Then goes software requirements specification. It’s the process of determining the needs and expectations of a new software product regarding a certain set of features that software engineers will need to develop.
Also, you need to keep in mind that in addition to the functional requirements, there will always be mandatory non-functional ones in your medical device software development. Mostly, these requirements are defined by the software architecture design or regulatory compliance needs.
-
Software architecture design
This process concerns a high-level design of your software solution. In the case of medical software development, you need to have flexible and scalable software architecture. So your software partner will be able to quickly configure and change modules and adjust the system according to the actual needs.
-
UI/UX design
Nowadays, consumers’ expectations have run so high that user-friendly interfaces and good-lookingness are a must. Or the users just leave. According to the Forrester report, a well-thought-out UI may boost the conversion rate of your product by up to 200%.
That’s why for a successful medical product, it’s necessary to create a user-friendly interface and test user scenarios.
-
Product (or MVP) development
As obvious as it gets, the software engineering team builds the infrastructure, front-end, and back-end parts of the solution at this stage. We’ve already mentioned that the app needs to be user-friendly, but performance is no less important.
Delivering a high-performing SaMD can be challenging, so many medical device software developers opt for the agile software development life cycle. Agile methodology helps to focus on quality and speed by introducing incremental processes.
If you want to know more about agile development, you can read an article by the Vilmate CTO, Igor Bagayev: Pros and Cons of Agile Approach: CTO’s Perspective.
-
Quality assurance
Software testing plays a huge role in medical device development. There are many types of testing, each with its own set of goals and methodologies. You can find more information on our dedicated QA & Testing service page.
When it comes to SaMD, regulatory requirements make software verification and validation (V&V) a crucial point for product development.
Software verification examines the solution and its accompanying documentation for consistency, completeness, and correctness. And software validation is a confirmation of precise requirements for a particular intended application. In layman’s terms, these processes can be described in the following way:
Verification: Are we building the product right?
Validation: Are we building the right product?
-
Maintenance
An essential stage of medical device software development is software maintenance. It’s the process of upgrading, modifying, and updating software to stay up with client demands. After a product has been released, software maintenance is performed for various purposes, including enhancing the software overall, addressing issues or bugs, increasing performance, and more.
So in the case of SaMD development, we will need to integrate it with various smart devices and provide updates regularly.
There’re many devices that can be integrated into Software-as-a-medical device. But the best option is considered to be a smartwatch because of its special control sensors. It can also be smartphones, wearable fitness trackers, PCs, laptops, or even smart TVs.
We’ve discussed the theory, but now, we can get down to business. So let’s dive into an example of a Software-as-a-medical device.
Health data in one app: ImagineCare
ImagineCare was designed as the platform of the future, which we all want to be healthier and safer. This medical device software was created to help patients and doctors to establish communication.

The application collects and analyzes data received from sensors of special diagnostic medical devices. It’s especially useful for people who suffer from chronic diseases or have unique requirements.
ImagineCare wants to give people a sense of security and complete control over their health. And the Vilmate team has helped ImagineCare to turn this idea into a successful product.
Final words
We’ve discussed how medical device software is created, who needs it, and why. SaMD is a promising direction in software development because people will always care for their health. With its handy technologies, the medical device industry can make it much easier.
But the process of Software-as-a-medical device product development is not so easy because of regulatory requirements.
If you need a reliable software partner to start your medical device software development project, you can contact us. For more examples from the Vilmate team, you can visit our Portfolio.




